pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
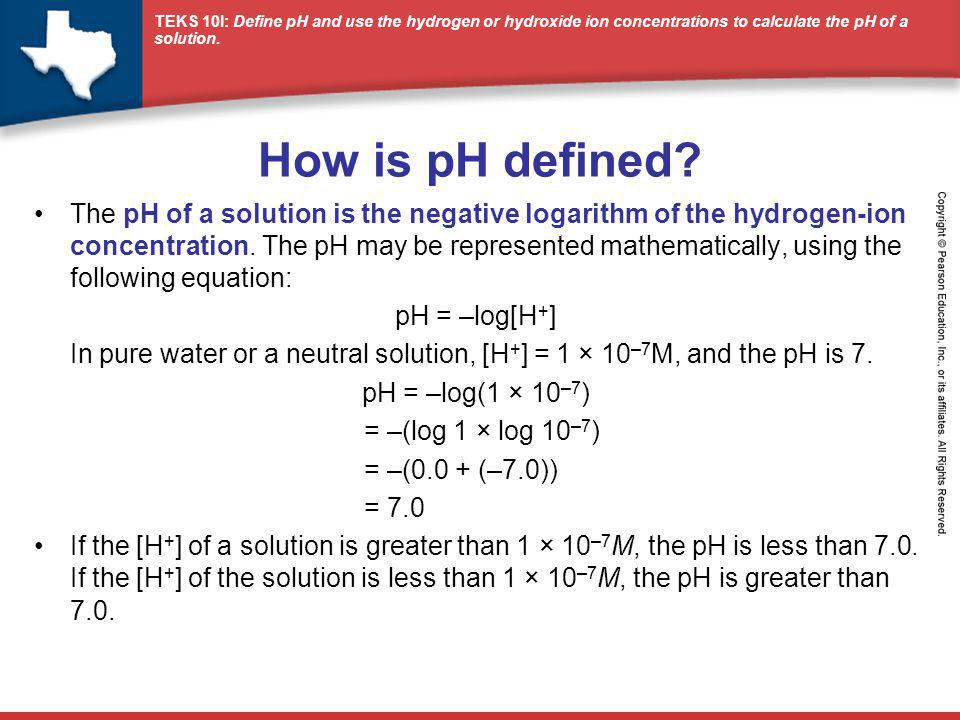
How is pH defined? The pH of a solution is the negative logarithm of the hydrogen-ion concentration. The pH may be represented mathematically, using the. - ppt video online download
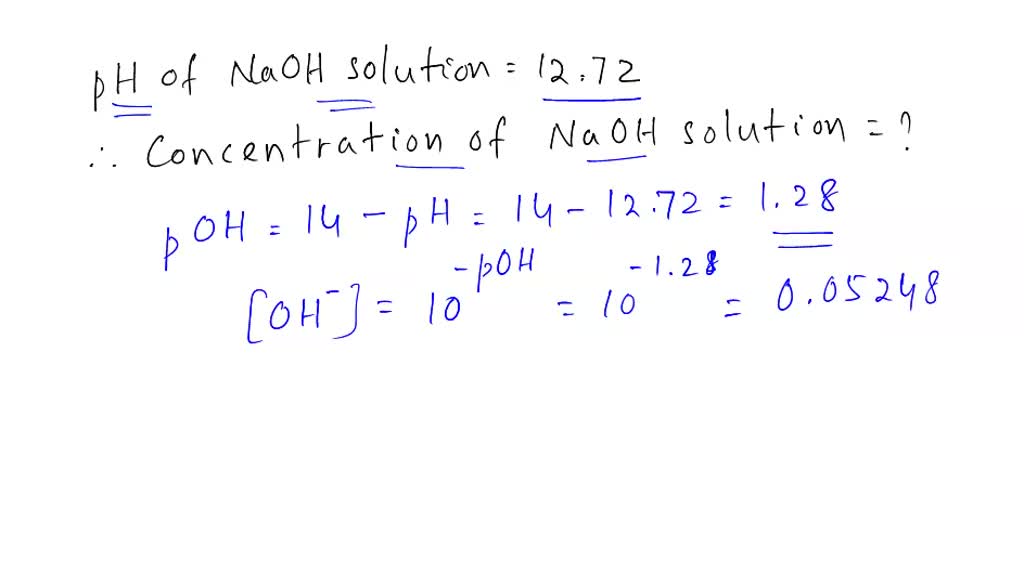

![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)



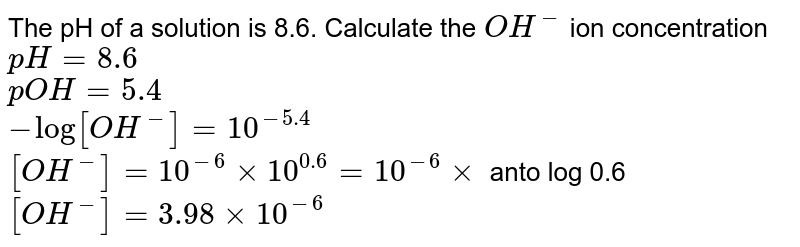




![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)
![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)




:max_bytes(150000):strip_icc()/litmuspaper-56a129a23df78cf77267fd9f.jpg)