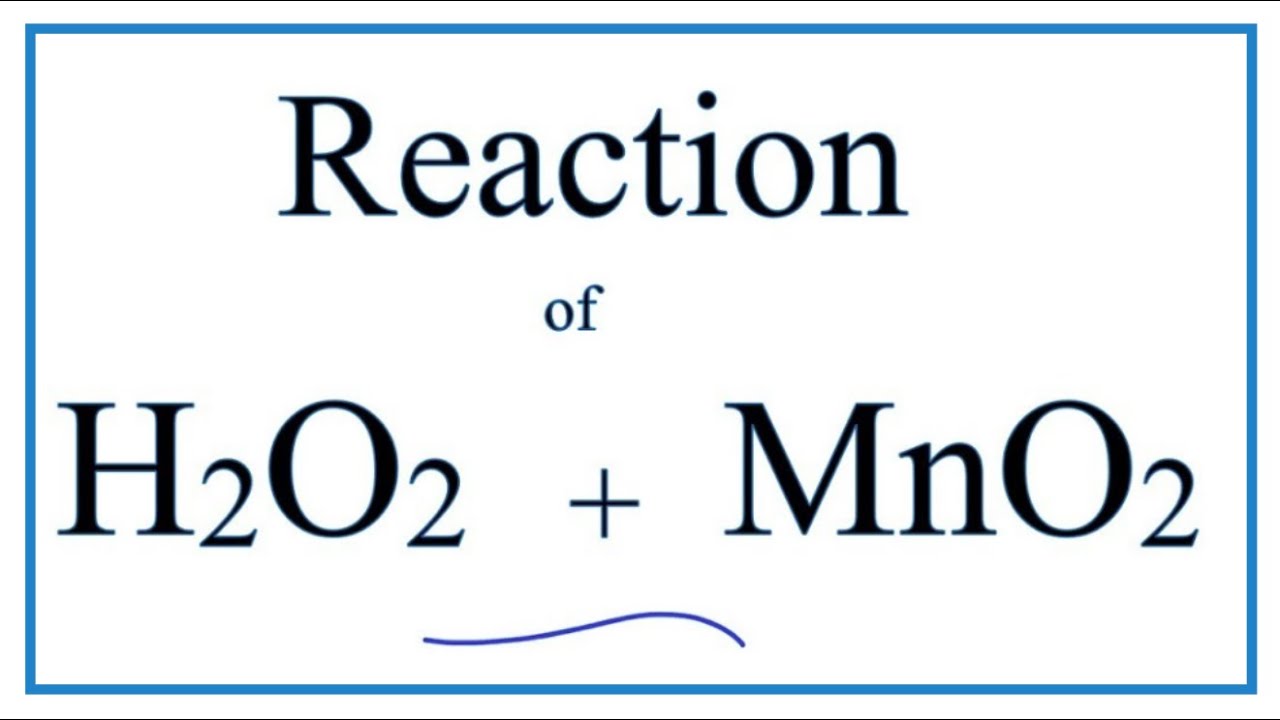IA on effectiveness of different types of catalysts MnO2 vs Fe(NO3)3 on the rate of decomposition of H2O2 measured using a pressure sensor.
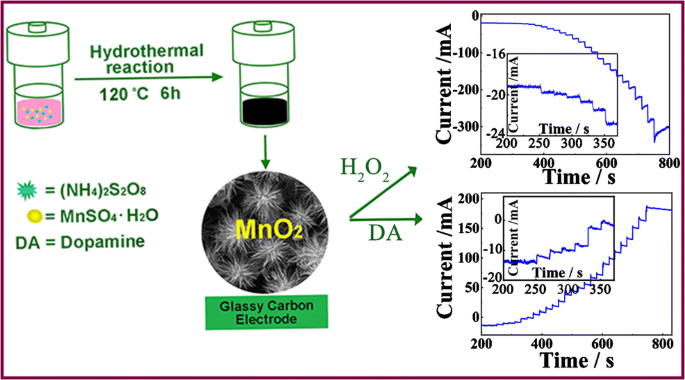
A hollow urchin-like α-MnO2 as an electrochemical sensor for hydrogen peroxide and dopamine with high selectivity and sensitivity | SpringerLink

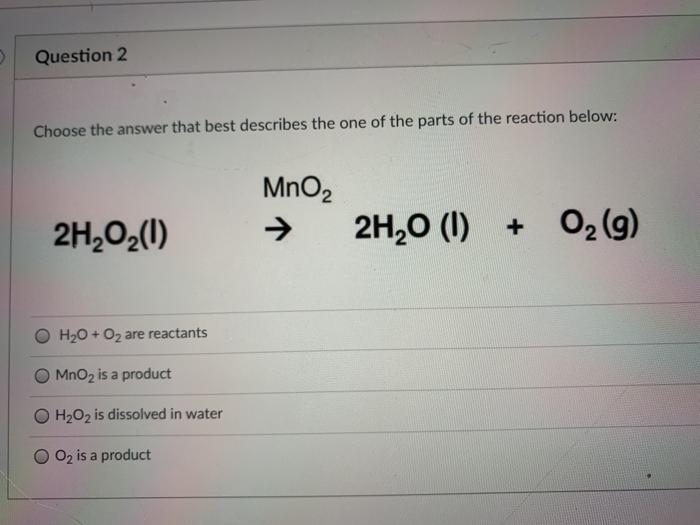
Question Video: Identifying the Correct Statement For the Decomposition of Hydrogen Peroxide Using a Manganese Dioxide Catalyst | Nagwa
Thermocatalytic Behavior of Manganese (IV) Oxide as Nanoporous Material on the Dissociation of a Gas Mixture Containing Hydrogen

Determination of H2O2 by MnO2 modified screen printed carbon electrode during Fenton and visible light-assisted photo-Fenton based removal of acetamiprid from water - ScienceDirect

inorganic chemistry - Reaction intermediates of MnO2 catalyzed H2O2 decomposition reaction - Chemistry Stack Exchange

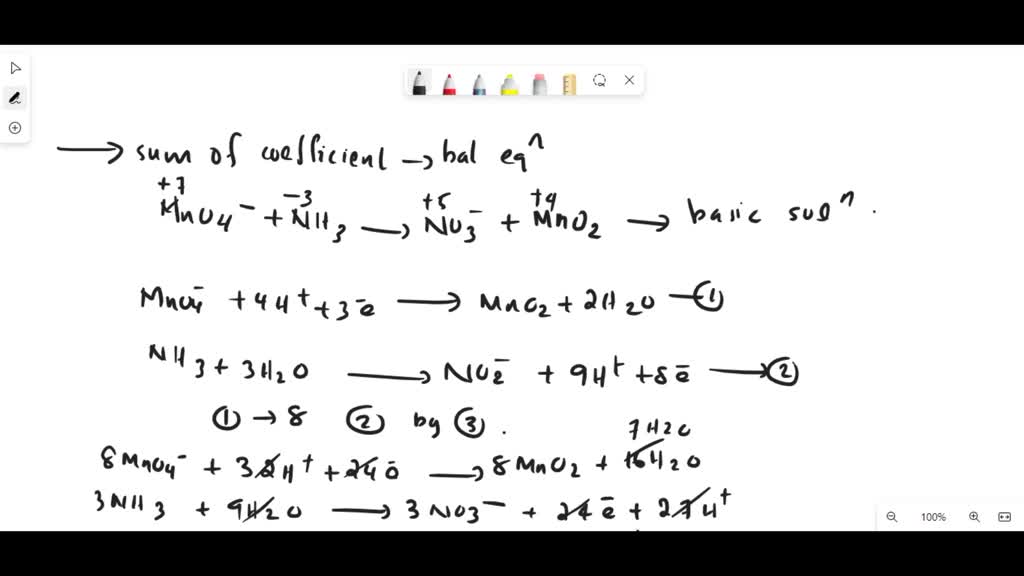
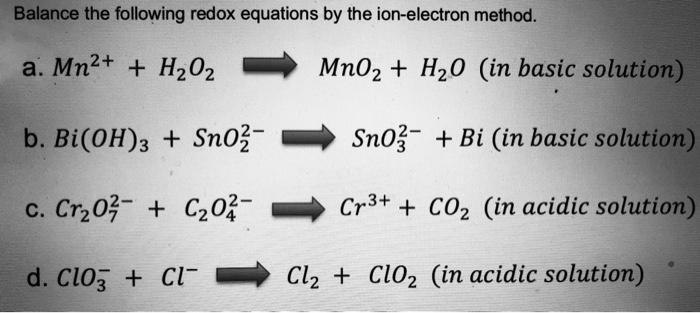
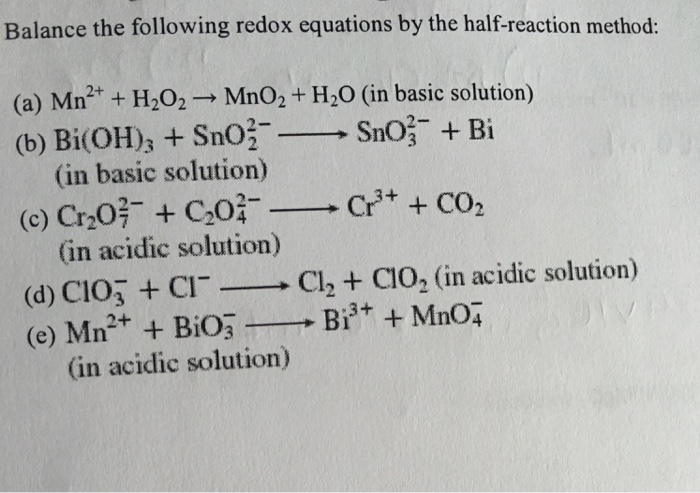
SOLVED: Mn2+ + H2O2 → MnO2 + H2O in basic medium Separate the reaction into two half-reactions and balance each of them.

In the following reaction: SO2(g) + 2H2S(g)→ 3S(s) + 2H2O(l) , the number of moles sulphur formed by 2 moles each of SO2 and H2S is :

K956: Catalysis – MnO2 catalyzed decomposition of H2O2 (“Genie in a Bottle”) | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder